
Note: The current system of foods with functional claims (FFC) is summarized in a leaflet in English.
The Consumer Affairs Agency (CAA) recently solicited opinions on the Cabinet Office Ordinance (Draft) (Japanese) to partially amend the Food Labeling Standards (Japanese) in accordance with the revision of the system for FFC. (from June 27 to July 26, 2024)
The point of the proposed amendment is to revise the definition of FFC (Article 2) and clarify its requirements. The draft also proposed to add the Appended Table 26 and 27 to the Standards, which would stipulate notification items for FFC and matters to be complied with after the notification.
Foods that do not meet these requirements are to be considered violations of Article 9 of the Food Labeling Standards under the Food Labeling Act.
Here is the summary of the new proposed requirements for FFC.
- The CAA will be able to prohibit the labeling of foods with no adequate scientific backing; in other words, those not recognized by the Commissioner of the CAA as appropriate for functional claims based on sufficient scientific knowledge.
- As matters to be complied with after notification, the following four items have been added.
1. Report any new scientific findings related to functionality and safety to the Commissioner of the CAA
2. Maintain quality control (compliance with Good Manufacturing Practice (GMP) for tablets, capsules, and other foods made from natural extracts)
3. Report information on any health hazards (limited to those diagnosed by a physician. Report it promptly even if the causal relationship with the food in question is unclear)
4. Report the results of self-checks of these above-stated compliance items to the Commissioner of the CAA annually
Public comments for the GMP standards (draft) (Japanese) were also solicited until Augst 16 and the content is briefly explained in the other article. - The notification items are based on what was stipulated in the Guidelines for submitting a notification of foods with function claims (Japanese) and further clarifies this stipulated content in accordance with the Cabinet Office Ordinance. The GMP standards for “tablets, capsules, and other foods made from natural extracts” have been newly established.
- The method of labeling the notification information on the package has also been reviewed to prevent misidentification as a pharmaceutical product, to differentiate it from Foods for Specified Health Uses, and to provide information on safety and functionality to consumers.
The main points of the revision of the labeling method on packages (Article 3, Appended Table 22) are as follows.
- The labeling for FFC shall be indicated in a frame at the top of the main surface.
- The notification number shall be indicated in a position close to the label of FFC.
- The [Reference Material 4] (Japanese) (the 74th meeting of the Food Labeling Subcommittee) gives easy-to-understand examples of the labeling on functional claims (Functionality of foods containing functional ingredients and the ingredients themselves, supported by scientific evidence) such as,
-It will no longer be allowed to indicate part of the functionality
-For functional ingredients of which the notification is based on a research review, indicate “it has been reported that XX (effects on health)”
The following is a proposed revised labeling example of food products for which notification was made based on the research review of functional ingredients in [Reference Material 4] (The example is for fresh foods, but the revised parts can be applied to other foods, too. Changes are in red).

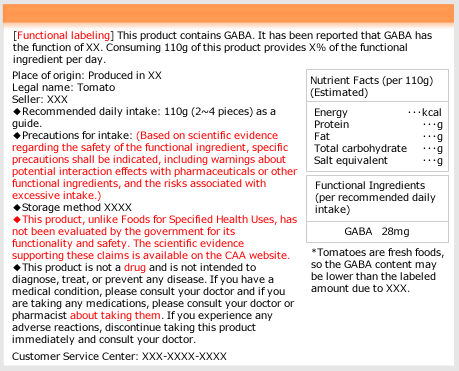
Lastly, the enforcement date is summarized in [Reference Material 3] titled Review of the FFC System and the enforcement date (Japanese) (Some of the revision draft will be enforced on September 1, 2024). The content of the draft may be slightly revised based on public feedback. Businesses that have notified FFC or plan to do so should pay close attention to future developments.
Share/Like/Follow:
Newsletter Signup
We issue monthly e-newsletters, which provide you with the latest updates on food labeling/regulations in Japan.
If you want to make sure to not miss any issue, please click below.
Related Service
Research Services on Ingredients & Food Labeling -For the Japanese Market-
We verify the conformity of ingredients and additives with the standards for use in Japan based on specifications such as formulation lists. We also verify the conformity of the proposed labeling of ingredient names, nutrients, etc. with the labeling standards based on specifications such as formulation lists.
Label bank Co., Ltd. Regulatory inspections and Consulting Research staff
Born in Japan. Has a long experience in the food manufacturing industry. He is engaged in research work on both domestic and imported food ingredients and additives as well as provides consulting services on food standards, additives compliance, and food labeling.






