On July 12, 2024, the Consumer Affairs Agency (CAA) began soliciting opinions on the standards for the manufacture or processing of tablets, capsules, and other Foods with Functional Claims (FFC) that Use natural extracts as ingredients (draft) (Japanese). The public comment was closed on August 16th.
The draft notice is based on the decision made at the ministerial meeting onFuture Measures Regarding FFC Labeling System in response to the Incident Involving Red Koji Supplements, where it was decided that for supplements with functional claims, manufacturing management based on GMP (Good Manufacturing Practices) will be a compliance requirement for the notifier under the Food Labeling Standards. The content of the draft follows Annex 2 (Japanese) of the GMP Guidelines (No. 0311 No. 2, March 11, 2024) (Japanese)The draft notice (Japanese) as well as the GMP guidelines also provides the definitions of certain terms (Article 2). Here are some excerpts. The provisions regarding the responsibilities of the notifier and the creation and management of documents and records have also been stipulated.
- “Ingredients” mean all the ingredients used in the manufacturing of a product.
- “Source materials” mean animals and plants, or specific parts thereof, microorganisms, chemicals, minerals and other substances used to manufacture ingredients.
- “A Product“ means a food product that has completed all processes such as manufacturing.
- “Intermediate products” mean items produced during the intermediate stages of product manufacturing.
- “Equivalents to ‘a Product’” include ingredients, containers and packaging, products, and intermediate products.
- “Lot” means a group of products manufactured to have homogeneity through a series of processes within a single manufacturing period. (Hereafter abbreviated)
The following image explains the scope of GMP ([Reference Document 5-1] the Scope of GMP Notification (the 74th Food Labeling Subcommittee))(Japanese).
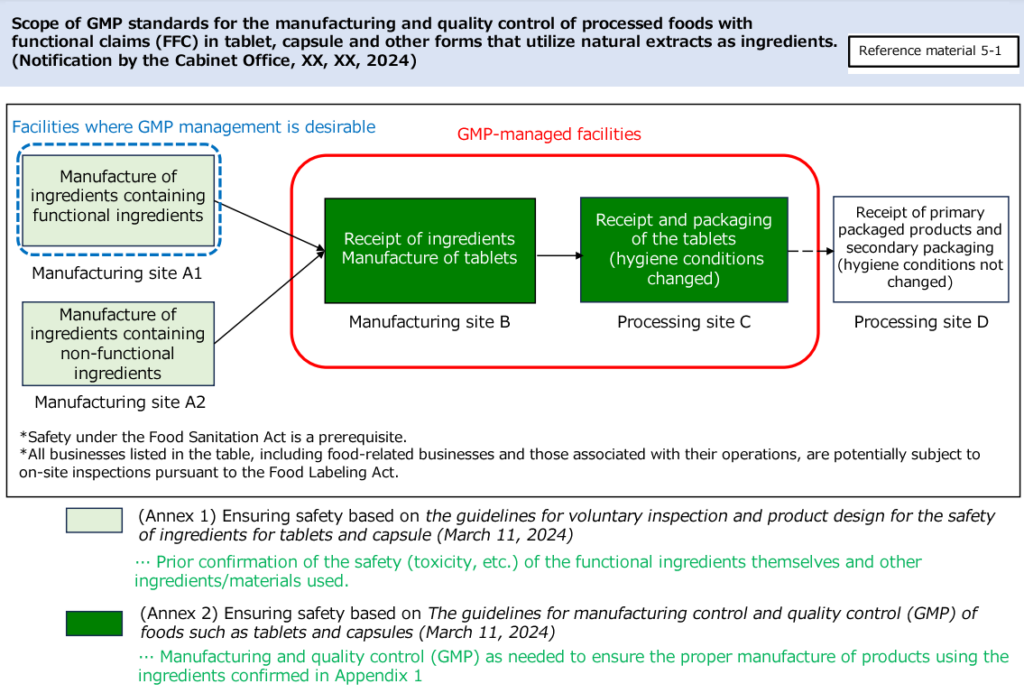
The enforcement date is scheduled on September 1, 2024. This draft notification covers the contents of Annex 2 (related to manufacturing site) in the above figure.
Regarding the contents of Annex 1 (related to ingredients), it is expected that a draft notification will be compiled in the future.
Share/Like/Follow:
Newsletter Signup
We issue monthly e-newsletters, which provide you with the latest updates on food labeling/regulations in Japan.
If you want to make sure to not miss any issue, please click below.
Related Service
Research Services on Ingredients & Food Labeling -For the Japanese Market-
We verify the conformity of ingredients and additives with the standards for use in Japan based on specifications such as formulation lists. We also verify the conformity of the proposed labeling of ingredient names, nutrients, etc. with the labeling standards based on specifications such as formulation lists.

Label bank Co., Ltd. CEO (Founder)
Born in Japan. Working on solving various issues related to food labeling operations. Also regularly gives lectures for various organizations in Japan.
Co-authored ‘A Practical Guidebook to Food Labeling for Export – from scratch‘ and ‘2nd Revised Edition: Guidebook Food Labeling Law and related business practical points – from scratch‘ (DAI-ICHI HOKI CO., LTD).