
On September 28, 2022, Food and Drug Administration (FDA) issued a proposed rule to update the definition of the nutrient content claim “healthy”, which was set in 1994. The existing definition has limits for total fat, saturated fat, cholesterol and sodium and to qualify, foods must also provide at least 10% of the Daily Value (DV) for one or more of the following nutrients: vitamin A, vitamin C, calcium, iron, protein and fiber. The FDA accepted public comments until December 28, 2022.
Background of the revision
The latest dietary guidelines in the US have emphasized balanced dietary patterns to consume nutrients rather than focusing on individual nutrients contained in foods. In other words, one of the reasons for this revision is that there is a discrepancy between the current definition of “healthy” and the latest concept of it.
Claims such as “healthy” are an important source of information that allows consumers to select healthier foods at a glance. Currently, more than 80 percent of Americans are estimated to exceed the recommended intake limits for added sugars, saturated fats, and sodium while consuming less in vegetables, fruits, and dairy products. To help consumers improve nutritional and dietary balance and reduce the burden of chronic diseases, the FDA is proposing the changes as a part of an effort to improve health equity in line with current nutritional science and dietary guidelines.
The framework for “healthy”
As a revised proposal, total fat and cholesterol are considered to be removed, and added sugars are to be added among the nutrients covered by the current standards. The proposed definition of “healthy” is based on the revised Nutrition Facts and current nutrition science and Federal dietary guidance, “the Dietary Guidelines for Americans, 2020-2025”, for consumers to maintain healthy dietary practices close to the recommended dietary standards.
Specifically, the proposed definition of “healthy” would require food products contain a certain amount of food from at least one of the food groups or subgroups recommended by “the Dietary Guidelines, 2020-2025” in order to be labeled “healthy”. Limits on added sugars, saturated fat and sodium are set based on Daily Value (DV).
For example, fruits and vegetables, grains, protein, dairy products, etc. must be contained in a certain amount or more, and raw, whole fruits and vegetables automatically qualify for use of the claim.
Also, foods that currently do not meet the “healthy” claim criteria may still meet the requirements under the revised definition. e.g. water, avocados, nuts and seeds, higher fat fish such as salmon, and certain oils In contrast, foods with current “healthy” claims such as white bread, highly sweetened yogurt, and highly sweetened cereal may not meet the proposed definition.
Proposed Criteria for Certain Food Groups and Sample Foods
Per Reference Amount Customarily Consumed (RACC)
| Food Groups | Food Group Equivalent Minimum | Added Sugar Limit | Sodium Limit | Saturated Fat Limit |
|---|---|---|---|---|
| Grains | 3/4 oz whole-grain equivalent | 5% DV (2.5 g) | 10% DV (230 mg) | 5% DV (1 g) |
| Dairy | 3/4 cup equivalent | 5% DV (2.5 g) | 10% DV (230 mg) | 10% DV (2 g) |
| Vegetable | 1/2 cup equivalent | 0% DV (0 g) | 10% DV (230 mg) | 5% DV (1 g) |
| Fruit product | 1/2 cup equivalent | 0% DV (0 g) | 10% DV (230 mg) | 5% DV (1 g) |
| Food Groups/Proteins (examples) | Food Group Equivalent Minimum | Added Sugar Limit | Sodium Limit | Saturated Fat Limit |
|---|---|---|---|---|
| Game meat | 1 ½ oz equivalent | 0% DV | 10% DV | 10% DV |
| Seafood | 1 oz equivalent | 0% DV | 10% DV | 10% DV |
| Nuts and seeds | 1 oz equivalent | 0% DV | 10% DV | 5% DV* |
* Excluding saturated fat derived from nuts and seeds
| Food Groups/Oils (examples) | Food Group Equivalent Minimum | Added Sugar Limit | Sodium Limit | Saturated Fat Limit |
|---|---|---|---|---|
| 100% Oil | N/A | 0% DV | 0% DV | 20% of total fat |
| Oil-based Spreads | N/A | 0% DV | 5% DV | 20% of total fat |
| Oil-based Dressing* | N/A | 2% DV | 5% DV | 20% of total fat |
* Must contain at least 30% oil and saturated fat level of the oil must be ≤ 20 percent of total fat
| Sample Foods | Individual food | Mixed product | Meal |
|---|---|---|---|
 |  |  | |
| Amount of food groups required | 6-oz yogurt (1 food group equivalent)* | 1/8 cup dried fruit and 1/4 oz nuts (At least 1/2 food group equivalent each from 2 different food groups) | 1 oz salmon, 1/2 cup green beans, 3/4 oz brown rice (At least 1 food group equivalent each from 3 different food groups) |
| Nutrients to Limit (no more than)** | 2 g saturated fat 230 mg sodium 2.5 g added sugar | 1 g saturated fat*** 230 mg sodium 0 g added sugar | 4 g saturated fat 690 mg sodium 2.5 g added sugar |
* A food group equivalent is the amount of a food group required
** Amounts based on percentage of the Daily Value for that nutrient
*** Saturated fat from nuts/seeds does not contribute to limit
“Healthy” symbol
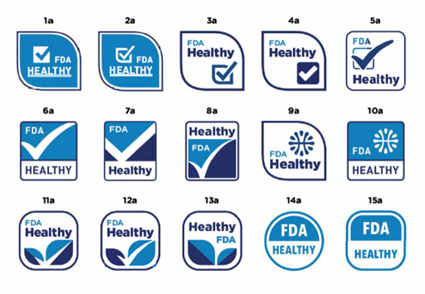
Other than the above, the FDA has begun to conduct research on a symbol that industry can voluntarily use to label food products that meet the proposed “healthy” definition. Symbols may be particularly helpful for those with lower nutrition knowledge to identify foods that can be the foundation of a healthy eating pattern. The results of the surveys were published twice in May 2021 and March 2022 through notices in the Federal Register.
Public comments on this issue are expected to be solicited, so keep an eye on the future move on the revision.
[the draft “healthy” symbols]
References
- Use of the Term Healthy on Food Labeling (FDA summary)
- Food Labeling: Nutrient Content Claims; Definition of Term “Healthy”
- FDA Proposes Updated Definition of ‘Healthy’ Claim on Food Packages to Help Improve Diet, Reduce Chronic Disease
- Appendix G Healthy Symbols Figure
- Dietary Guidelines for Americans, 2020-2025 and Online Materials
Newsletter Signup
We issue monthly e-newsletters, which provide you with the latest updates on food labeling/regulations in Japan.
If you want to make sure to not miss any issue, please click below.
Related Service
Research Services on Ingredients & Food Labeling -For the Japanese Market-
We verify the conformity of ingredients and additives with the standards for use in Japan based on specifications such as formulation lists. We also verify the conformity of the proposed labeling of ingredient names, nutrients, etc. with the labeling standards based on specifications such as formulation lists.

Label bank Co., Ltd. Regulatory inspections and Consulting Research staff
Born in Taiwan. Specializing in nutrition, she is mainly engaged in research work on ingredients and additives to be exported from Japan to overseas, as well as managing databases for legal search systems, such as nutrients and labeling standards in various countries.



