On July 29, 2025, the 1st Discussion on Japan’s Front of Package Nutrition Labelling (FOPNL) 2025 (in Japanese) was held and the guidelines for Japan’s FOPNL (draft) (in Japanese) were presented. As part of our on-going efforts to report on the situation, we would like to outline the labelling methods for Japan’s front of package nutrition labelling as presented in these draft guidelines (see our comments point by point below).
(1) Format
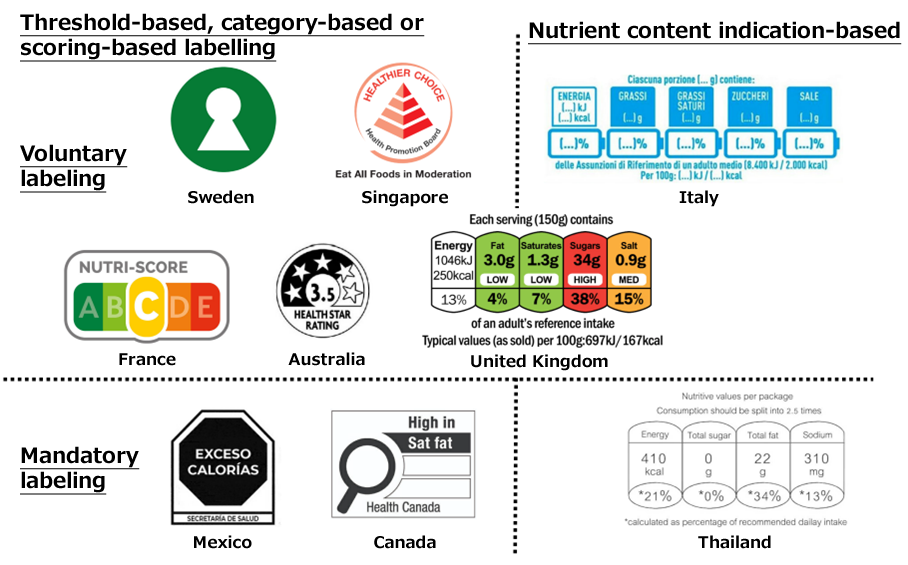
The format suggested for Japan’s FOPNL has been revealed and is as shown in Figure 1. For tall containers such as PET bottles, a vertical display will also be permitted, as shown in Figure 2.
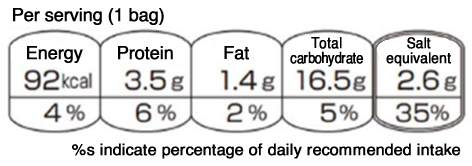
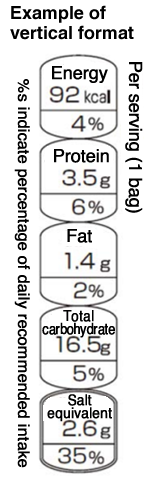
(2) Food Unit
The food unit shall be one serving of the food item, and its quantity (e.g., ○ pieces, ○ bags, ○ g) shall be displayed together. The display location shall, in principle, be the upper left corner of the FOPN labeling when displayed horizontally.
(3) Amount of Nutrients
The amount of nutrients will have to indicate the amount present in the edible portion as sold and should ideally match the values in the nutrition facts table.
However, for foods where nutrient values change between “as sold” and “as consumed” states, it is permissible to display the nutrient value on the Japan’s FOPNL as per the “as consumed” state. Please refer to “6. Handling of foods where nutrient values change between “as sold” and “as consumed” states (in Japanese)” for further details on the reasoning behind this.
(4) Amount (in %) compared to reference values for nutrients intakes
The reference values for nutrients intakes are calculated based on the recommended amounts in the Dietary Reference Intakes for Japanese (2025 Edition) (in Japanese) for adults aged 18 years and older.
Regarding the display of percentages compared to such reference values, the first decimal place is rounded to the nearest whole number. However, when rounding results in 0%, it is recommended to express it as “<1%” or “less than 1%”.
(5) Where to label and font/color used in the format
The FOPNL shall be located, in principle, on the front (main surface) of the food container packaging.
It will be desirable that the font size used be at least 8 points as specified in JIS Z8305 (1962) (or 5.5 points or larger when the displayable area is roughly 150 cm² or less). As for the color of the text and frames, it will have to be one contrasting with the color of the background – and displayed in a single color.
Beyond above topics, the mentioned draft guidelines also cover the scope of application as well as other matters. Furthermore, in addition to the topics that have already been discussed for a while, it now includes a Q&A on the guidelines for Japan’s FOPNL (in Japanese).
While the FOPNL is planned to be voluntary, it is a new labeling system that will contribute to maintaining and promoting consumer health. Therefore, we encourage our readers to consider its implementation.
Note on future steps: Public comments will soon be solicited, and the second discussion is scheduled to be held from October (or later) this year.
Share/Like/Follow:
Newsletter Signup
We issue monthly e-newsletters, which provide you with the latest updates on food labeling/regulations in Japan.
If you want to make sure to not miss any issue, please click below.
Related Service
Research Services on Ingredients & Food Labeling -For the Japanese Market-
We verify the conformity of ingredients and additives with the standards for use in Japan based on specifications such as formulation lists. We also verify the conformity of the proposed labeling of ingredient names, nutrients, etc. with the labeling standards based on specifications such as formulation lists.
Label bank Co., Ltd. Regulatory Review and Development
Born in Japan. Specializing in nutrition, she is engaged in research work on ingredients and additives imported to Japan from overseas, and provides consulting services on food standards, additives, and food labeling.