On March 9, 2023, the Consumer Affairs Agency(CAA) announced revisions to “Food Labeling Standards”, “Regarding Food Labeling Standards”, and “Food Labeling Standards Q&A”. The main revisions include the mandatory labeling of allergens for walnuts and the addition of rapeseed producing EPA / DHA* to the list of specified genetically modified agricultural products**. In this article, we would like to organize the details of the mandatory allergen labeling for walnuts as follows.
(*Please refer to past article for the information on the addition of rapeseed producing EPA / DHA to the list of specified genetically modified agricultural products.)
(**Agricultural products whose composition, nutritional value, etc. are significantly different from those of regular agricultural products because they were produced using recombinant DNA technology. See in the reference “Old and New Comparison table (Food Labeling Standards Q&A(the 15th revision)” pg. 24))
The main points of the revision of “Food Labeling Standards”
Walnuts were added to Appended Table 14, and the number of specified raw materials (mandatory labeling items for allergens in Japan) was changed from seven to eight.
| Before the revision (Old) | After the revision (New) |
|---|---|
| Shrimp Crab Wheat Soba (Japanese buckwheat) Egg Milk Peanut | Shrimp Crab Walnut Wheat Soba (Japanese buckwheat) Egg Milk Peanut |
The main points of the revision of “Regarding Food Labeling Standards”
Walnut was removed from the list of “specified raw materials equivalents”(recommended labeling items for allergens in Japan) and the number of items on the new list was changed from 21 to 20 items.
| Before the revision (Old) | After the revision (New) |
|---|---|
| Almond, Abalone, Squid, Salmon roe, Orange, Cashew nuts, Kiwifruit, Beef, Walnut, Sesame seed, Salmon, Mackerel, Soybean, Chicken, Banana, Pork, Matsutake mushroom, Peach, Yam, Apple, Gelatin | Almond, Abalone, Squid, Salmon roe, Orange, Cashew nuts, Kiwifruit, Beef, Sesame seed, Salmon, Mackerel, Soybean, Chicken, Banana, Pork, Matsutake mushroom, Peach, Yam, Apple, Gelatin |
The description of Appended Table 1 (the scope of the definition of specified raw materials) remains unchanged. However, “the scope” of the definition of walnut has been added to Food Labeling Standards Q&A (see below *1).
| Specific raw materials, etc. | Classification Number (1) | Classification Number (2) | Broad classification | Medium classification | Small classification |
|---|---|---|---|---|---|
| Walnut | 69 | 8591 | Drupe-like nuts | Other drupe-like nuts | Walnut |
No labeling examples have been added to Appended Table 2 (Examples of labeling for additives derived from specified raw materials, etc. ), same as before the revision.
| name of specified raw materials | Classification | Additive | labeling of specified raw materials | Remarks |
|---|---|---|---|---|
| Walnut | – | – | – | – |
No labeling examples have been added to Appended Table 3 (substitute declarations for specified raw materials and so on), same as before the revision.
| specified raw materials (items stipulated in Food Labeling Standards) | Substitute description | Extended description |
|---|---|---|
| A different description substituting the original by how it is described or words used, but can be understood to correspond to the same specified raw material. | Examples of descriptions that include the names of specified raw materials or alternative descriptions so that they can be understood as food products using these ingredients. | |
| Walnut | Walnut | Walnut bread, Walnut cake |
“2.2 Qualitative testing method” now includes the additional descriptions of real-time PCR method and nucleic acid chromatography.
| Before the revision (Old) | After the revision (New) |
|---|---|
| Qualitative testing methods include western blotting method and PCR method. Generally, western blotting method is used for egg and milk. As for wheat, buckwheat, shrimp, crab, and peanut, PCR method is commonly used. It is acceptable to use a qualitative testing method other than western blotting method or PCR method, however, it shall have the same or more accuracy than those methods. (Omitted) | Qualitative testing methods include western blotting method, PCR method, real-time PCR method, and PCR-nucleic acid chromatography. Generally, western blotting method is used for egg and milk. On the other hand, – PCR method is use for shrimp and crab – PCR method or real-time PCR method is used for wheat, buckwheat, and peanut – Real-time PCR method or nucleic acid chromatography is used for walnut It is acceptable to use a qualitative testing method other than western blotting method, PCR method, real-time PCR method, and PCR-nucleic acid chromatography, however, it shall have the same or more accuracy than those methods. (Omitted) |
In relation to this addition, the details of the Qualitative testing methods have been added and the “About the judgment tree” has been changed. If you would like to confirm the test method, you can check them.
The main points of the revision to “Food Labeling Standards Q&A”
Food Labeling Standards Q&A (D-3) regarding the scope of walnut (*1) has been added.
(D-3) What is the scope of “walnut” as specified raw materials?
(Answer) The term “walnut” refers to walnuts stipulated in the Japan Standard Commodity Classification Number 698591, and in addition to the overseas species (Chandler, Howard, etc.) that are mainly distributed, Japan’s species (Amigurumi, Shiurim, Himegurumi, and so on) are also subject to labeling.. Please note that walnut oil, walnut butter (and so on) are also allergens.
Although not included in this article, some additions and changes have been made to Food Labeling Standards Q&A in relation to the “Addition of rapeseed producing EPA / DHA as a specified genetically modified agricultural product (addition to Appended Table 18)”. In addition, Food Labeling Standards Q&A on how to label the Nutrition Facts for “foods sold and usually consumed together in sets”(e.g. a set product of undo (Japanese) noodle and soup) has been added. Please check if you handle related food products.
Period for transitional measures and upcoming schedules
As shown below, the period is until the end of March 2025.
The labeling for processed foods (excluding processed foods for business use) which are manufactured, processed, or imported from the date of enforcement of this the Cabinet Office Ordinance to March 31, 2025 and processed foods for business use which are sold by March, 31, 2025 shall remain applicable notwithstanding the provisions of Appended Table 14 of the Food Labeling Standards revised by said revised provisions.
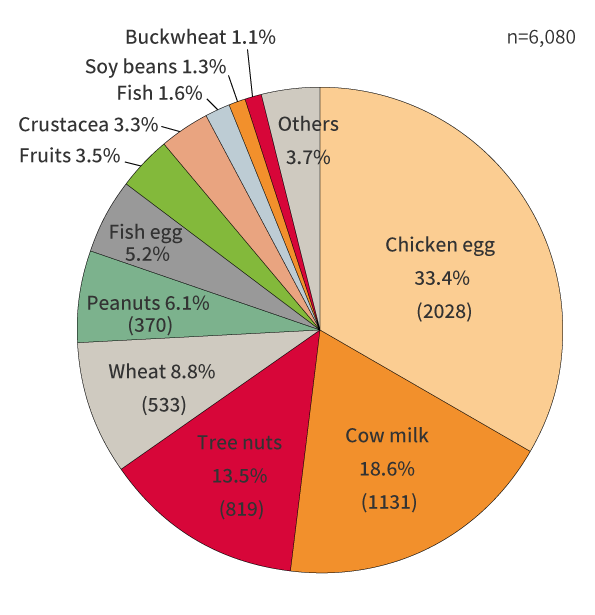
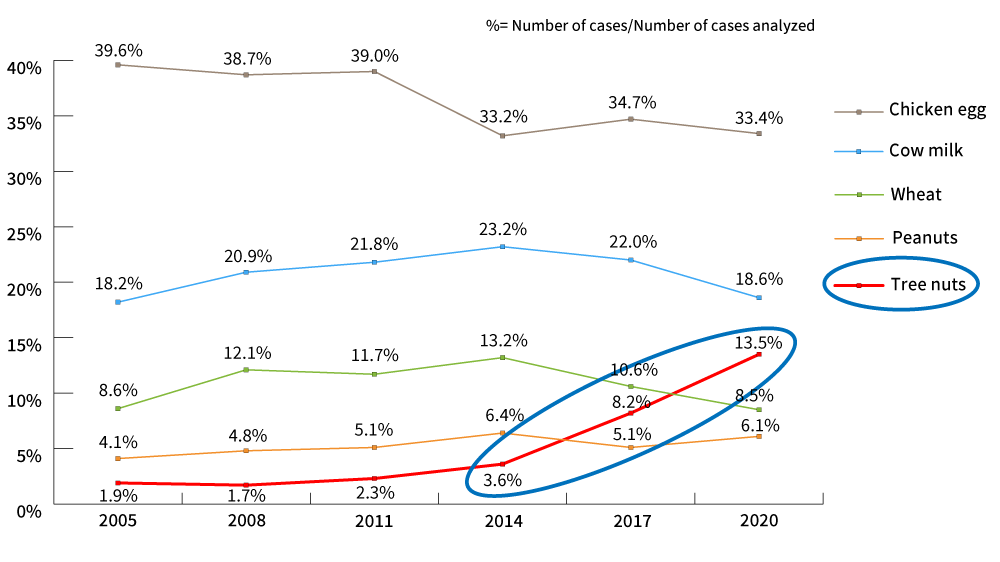
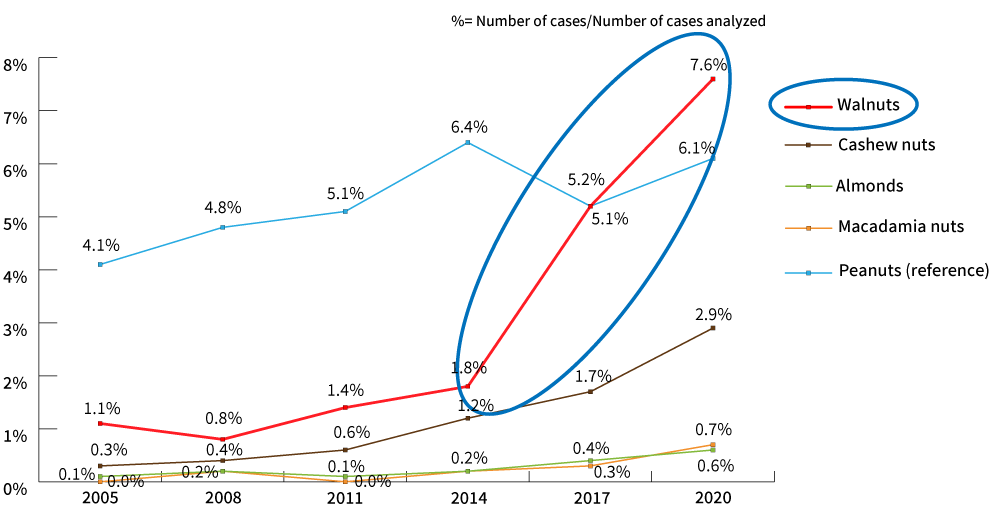
The revision was made in response to the 2018 Report on the Survey on food labeling regarding food allergy. The report revealed there has been a significant increase in health hazards caused by immediate-type allergies to nuts, particularly walnuts.
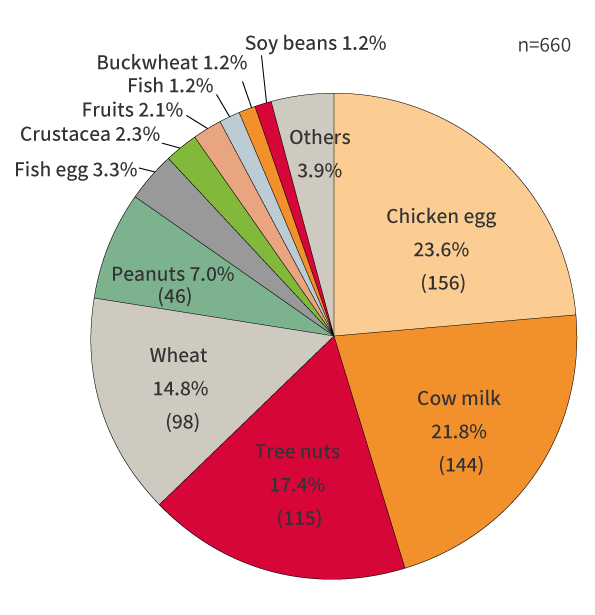
The survey conducted in 2021 revealed the cases of allergy have been on the rise since then. So, even during the period for transitional measures, it is important to reconfirm information management, such as ingredient specifications to be able to respond to inquiries from consumers.
References
- Food Labeling Act, etc. (Acts and Regulations and Centralized Information)
- Old and New Comparison table(Food Labeling Standards March 9, 2023 Cabinet Office Ordinance No. 15)
- Old and New Comparison table (Regarding Food Labeling Standards(the 28th revision))
- Old and New Comparison table (Food Labeling Standards Q&A(the 15th revision))
Share/Like/Follow:
Newsletter Signup
We issue monthly e-newsletters, which provide you with the latest updates on food labeling/regulations in Japan.
If you want to make sure to not miss any issue, please click below.
Related Service
Research Services on Ingredients & Food Labeling -For the Japanese Market-
We verify the conformity of ingredients and additives with the standards for use in Japan based on specifications such as formulation lists. We also verify the conformity of the proposed labeling of ingredient names, nutrients, etc. with the labeling standards based on specifications such as formulation lists.

Label bank Co., Ltd. CEO (Founder)
Born in Japan. Working on solving various issues related to food labeling operations. Also regularly gives lectures for various organizations in Japan.
Co-author of ‘Latest edition: Guide book Food Labeling Law and related business practical points – from scratch (Japanese version only)’ (DAI-ICHI HOKI CO., LTD/2019).